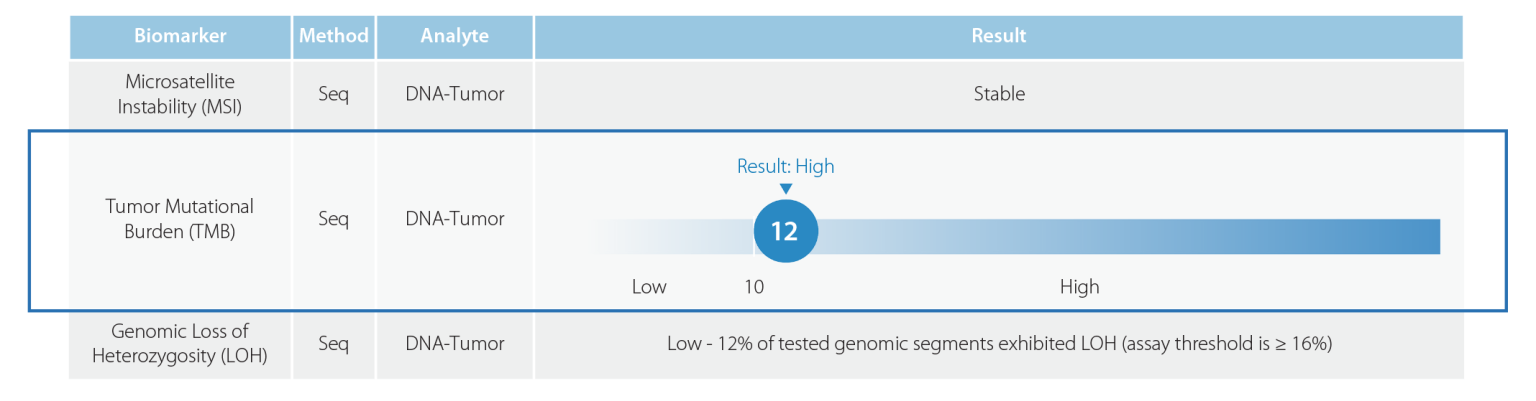
Tumor Mutational Burden
Tumor Mutational Burden
Non-synonymous mutations are changes in DNA that result in amino acid changes in the protein.1,2 The new protein changes result in new shapes (neo-antigens) that are considered to be foreign to the immune system.1,3 Immune checkpoint inhibitors are able to stimulate and allow the immune system to detect these neo-antigens and destroy the tumor.2 Germline (inherited) mutations are not included in Tumor Mutational Burden because the immune system has a higher likelihood of recognizing these alterations as normal.4
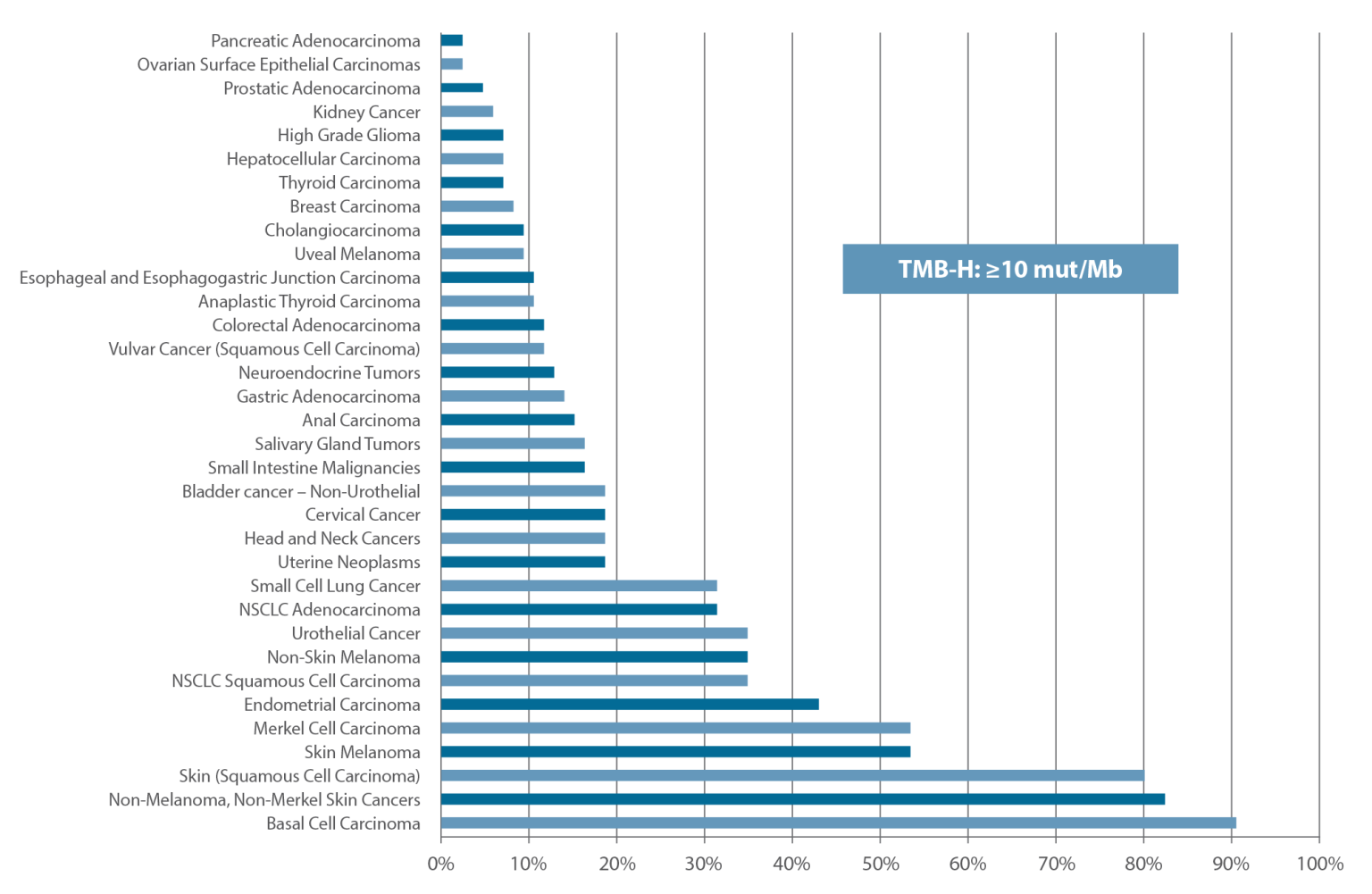
TMB has emerged as an important biomarker when considering immunotherapy in solid tumors. This is highlighted by the FDA accelerated approval of pembrolizumab (KEYTRUDA®) for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options. This approval is based on the results of the KEYNOTE-158 trial, which achieved an overall response rate of 29% (95% CI: 21, 39), with a 4% complete response rate and 25% partial response rate.5
Caris has collaborated with the Friends of Cancer Research TMB Harmonization Project to systematically characterize and standardize Tumor Mutational Burden testing and reporting to a common industry standard.6 Based on this collective work and exciting KEYNOTE-158 result and drug approval, Caris has updated the TMB high/low threshold to reflect greater than or equal to 10 mutations per megabase across all solid tumors, aligning the testing results to pembrolizumab for TMB-H cases.5
Caris Molecular Profiling TMB-H Cutoff Aligned Across All Solid Tumors
- Snyder A. N Engl J Med. 2014; 371:2189-2199. doi:10.1056/ NEJMoa1406498
- Le DT. N Engl J Med. 2015;372:2509-2520. doi:10.1056/NEJMoa1500596
- Rosenberg JE. The Lancet. 2016; 387(10031):1909-1920. doi:10.1016/S0140-6736(16)00561-4.
- Stewart TJ. Oncogene. 2008;27:5894-5903. doi:10.1038/onc.2008.268
- U.S. Food and Drug Administration. (2020, June 16). FDA approves pembrolizumab for adults and children with TMB-H solid tumors [Press release].
- Stenzinger, A, Allen, JD, Maas, J, et al. Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer. 2019; 58: 578– 588. https://doi.org/10.1002/gcc.22733
Caris Molecular Profiling Menu by Region
United States
Review and download the Caris molecular profiling testing menu for United States cases.
New York
Review and download the Caris molecular profiling testing menu for New York state cases.
International*
Review and download the Caris molecular profiling testing menu for International cases.
*Excluding EEA, EU, CH countries.
Caris Molecular Profiling Menu by Region
International & US
New York
European Union (EU)
Please complete the form below to have a Caris Scientific Rep (Molecular Science Liaison) contact you directly.
"*" indicates required fields
Order Profiling
Place an order today for a comprehensive, personalized Caris profiling report.