Homologous Recombination Deficiency
Homologous Recombination Deficiency (HRD)
HRD and Ovarian Cancer
- HRD-positive tumors may be sensitized to PARPi therapy.
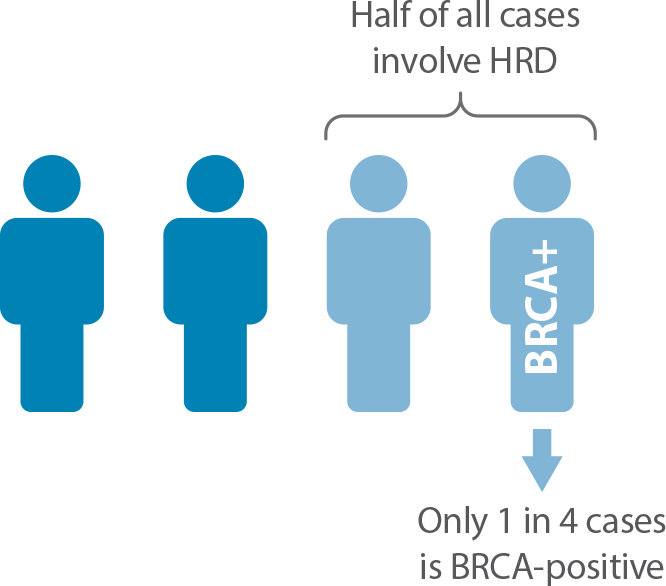
- HRD is present in nearly half of all ovarian cancer tumors.1
- HRD is often assessed solely on BRCA alterations, however BRCA alterations only account for half of HRD+ cases.2
- Non-BRCA causes of HRD include other gene alterations and epigenetic factors.
- Identifying HRD-positive tumors based on genomic scarring in addition to BRCA alterations results in a more complete assessment of HRD.
Genomic Scarring from HRD
Identifying gLOH and LST can assess HRD status from all causes, not just from BRCA alterations: |
 |
| gLOH | LST |
| Genomic Loss of Heterozygosity | Large-Scale State Transitions |
HRD Genes
BRCA1 and BRCA2 are key HRD genes, although others are also associated with HRD:| BRCA1 | CDK12 | PALB2 |
| BRCA2 | CHEK1 | RAD51B |
| ATM | CHEK2 | RAD51C |
| BARD1 | FANCL | RAD51D |
| BRIP1 | H2AX | RAD54L |
Caris HRD Status
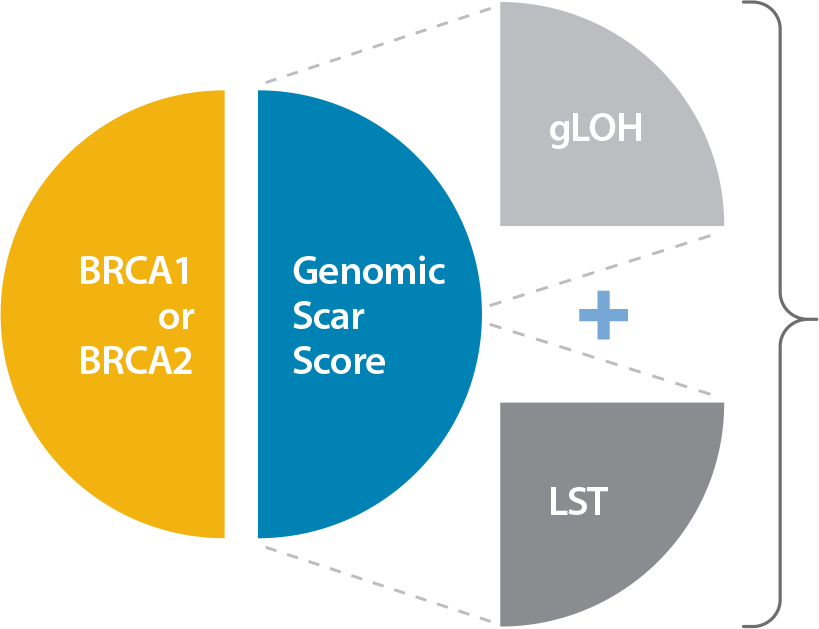
Caris reports HRD status based on BRCA1 or BRCA2 mutations and a Genomic Scar Score (GSS), comprised of genomic Loss of Heterozygosity (gLOH) plus Large-scale State Transitions (LST):
HRD-positive:
Either BRCA mutation detected or GSS = HIGH
HRD-negative:
BRCA mutation not detected and GSS = LOW
Reported for epithelial ovarian cancer cases (tissue profiling) at no additional cost or specimen requirements.
*Not available in all locations.HRD and PARPi Therapy
Homologous Recombination (HR) is a cellular process for maintaining chromosomal integrity. Alterations in one or several HR genes may result in HR Deficiency (HRD). Dysfunctional HR proteins cannot repair double-stranded DNA breaks, and other repair pathways such as poly-ADP-ribose-polymerase (PARP) or non-homologous end joining (NHEJ) cannot compensate, resulting in genome-wide errors and tumorigenesis.3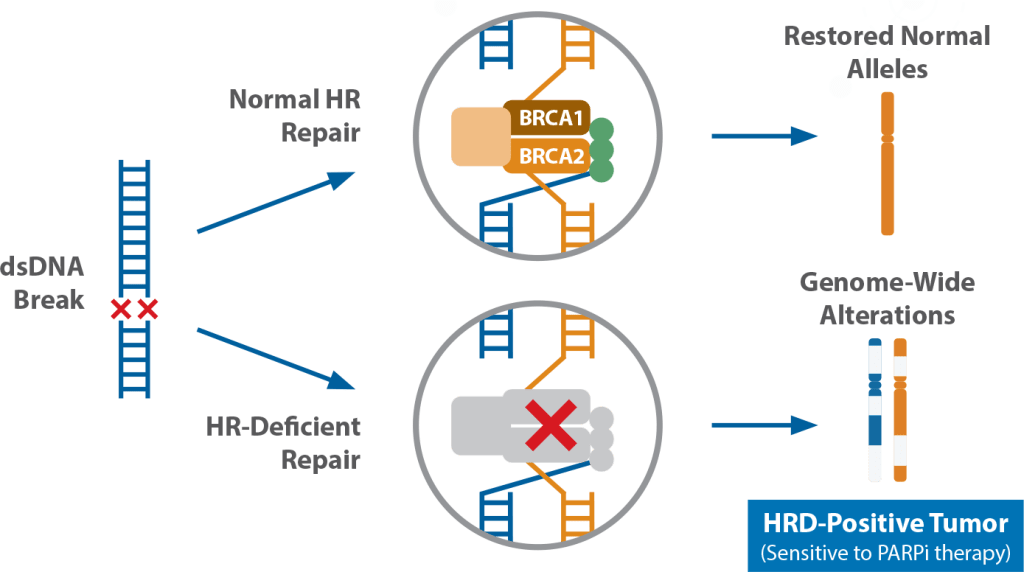
Figure 1. Homologous Repair Deficiency (HRD) is the result of genetic alteration(s) in HR genes (eg, BRCA1, BRCA2 and others) or epigenetic factors. Dysfunctional HR genes result in genome-wide errors and tumorigenesis.
HRD-positive tumors rely heavily on the PARP pathway for tumor growth and are therefore highly sensitized to PARP-inhibitor therapy, which specifically disrupts an already diminished DNA repair system in these tumor cells.
Caris Molecular Profiling Menu by Region
United States
Review and download the Caris molecular profiling testing menu for United States cases.
New York
Review and download the Caris molecular profiling testing menu for New York state cases.
International*
Review and download the Caris molecular profiling testing menu for International cases.
*Excluding EEA, EU, CH countries.
Caris Molecular Profiling Menu by Region
International & US
New York
European Union (EU)
Please complete the form below to have a Caris Scientific Rep (Molecular Science Liaison) contact you directly.
"*" indicates required fields
Easy to Interpret HRD Results
Results with Therapy Associations
| BIOMARKER | METHOD | ANALYTE | RESULT | THERAPY ASSOCIATIONS | BIOMARKER LEVEL* | |
|---|---|---|---|---|---|---|
| HRD** | Seq | DNA-Tumor | Positive | BENEFIT | niraparib, olaparib, rucanparib | Level 2 |
| BRCA1 | Seq | DNA-Tumor | Pathogenic Variant Exon 10 | p.I650fs | |||
* Biomarker reporting classification: Level 1 – Companion Diagnostics (CDx); Level 2 – Strong evidence of clinical significance or is endorsed by standard clinical guidelines; Level 3 – Potential clinical significance. Bolded benefit therapies, if present, highlight the most clinically significant findings.
** HRD – homologous recombination deficiency status. A positive result is indicative of a pathogenic or likely pathogenic variant(s) in BRCA1 and/or BRCA2 or high Genomic Scar Score (LOH + LST) which consists of genomic Loss of Heterozygozity (gLOH) + Large-scale State Transitions (LST).
Order Profiling
Place an order today for a comprehensive, personalized Caris profiling report.
1. Zhang H, Liu T, Zhang Z, et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell. 2016;166:755-765.
2. Sugino K, et al. Sci Rep. 2019;9[1]:17808; Pennington KP, et al. Clin Cancer Res. 2014;20[3]:764–775).
3. Ngoi NYL, Tan DSP. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: do we need it? ESMO Open. 2021 Jun;6(3):100144.