Right-In-Time Clinical Trials
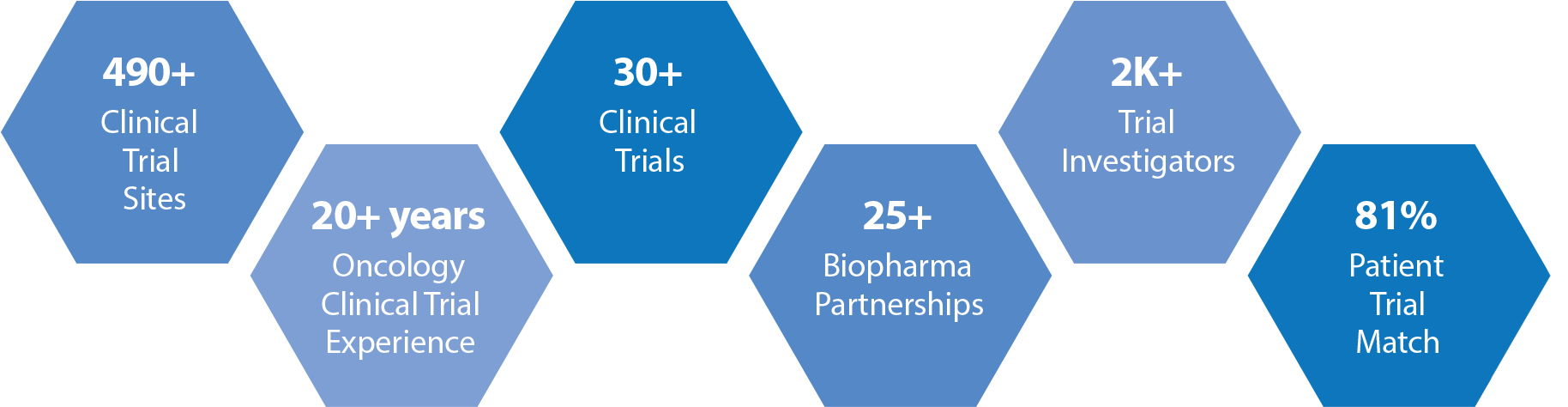
Right-In-Time Clinical Trial Network
How it Works
PATIENT-TRIAL MATCH PROCESS
![]()
Caris Comprehensive Genomic Profiling performed on patient.
![]()
Clinical Trial Navigator (CTN) determines patient-trial match. CTN contacts physician for matched RIT clinical trial.
![]()
Local Right-In-Time site activation.
![]()
Patient enrolled in local clinical trial.
DAY 1
DAY 2
DAYS 3-12
DAYS 13-14
RIT Network Registration
- Master CDA
- Master Clinical Research Agreement
- Core Regulatory Documents
- Standardized Site Qualification
Continuity of Care Matters
Studies have demonstrated that continuity of care has a direct positive correlation to outcomes and improved quality of clinical care. Patients within the Caris RIT oncology network gain access to innovative molecular diagnostics while remaining in the care of their current treatment team, locally. Continuity of care assists in eliminating the added stressors of extended travel times to clinical trial sites and unfamiliarity of new health care providers.
The Caris Difference - White-Glove Trial Matching
Patient matching is conducted with our Caris Clinical Trial Navigators (CTNs). CTNs are trained registered nurses specializing in oncology clinical trials. Through an in-depth review of inclusion and exclusion criteria, CTNs determine if a patient matches a clinical trial. If a patient isn’t ready to enroll in a clinical trial when the CTN speaks to the physician, the CTN can work with the oncologist to track patients until progression and when the clinical trial therapy is needed.
In addition to sites reviewing and selecting RIT trials through the TrialPlus+ platform, Caris CTNs will also notify oncologists directly when trial match is found through Caris molecular profiling. Once a match is confirmed, the CTN contacts your Caris representative for patient enrollment.
TrialPlus+
Physicians in the Caris RIT Network have the added benefit of accessing the TrialPlus+ database to do an on-demand search of new or existing clinical trials that may benefit a patient. TrialPlus+ is a proprietary clinical trials platform that allows research teams to download clinical trial protocols, confirm patient eligibility for appropriate RIT studies, and provide direct contact to the clinical trial manager. Having clinical trials on-demand in TrialPlus+ connects physicians to clinical trials to deliver breakthrough cancer medicines to their patients.
- Patient Centric Care
- Increased Access to Emerging Precision Therapies
- Continuity of Care Remains in Place
- Automated Patient-Clinical Trial Matching
- Patients Receive Treatment Fast